(KNSI) – COVID vaccines will soon be available for infants and toddlers in central Minnesota. The rollout will be measured in days, not hours, however. CentraCare lead COVID-19 Physician Dr. George Morris says there is still some planning that needs to occur within the organization.
“We don’t have any shipment to give today on hand. So, we will be getting it and then developing the processes and documentation in our system that we need. I would think that within one or two weeks we will be ready to give it to people here in central Minnesota,” he says.
Even though vaccines for the youngest age groups have not been available until now, expected demand is muted. Morris says the best case scenario he sees as far as adoption rates are concerned is for one-fourth of children under five to be immunized.
“Probably 10-25 [%] is where I’d ballpark it. And it will depend a little bit on communities. You know, if you want to view it as a national percentage, a Minnesota percentage, and then what we’ll see here in central Minnesota.”
Clinical trials yielded very different results for the vaccines from Moderna compared to Pfizer. Moderna Chief Medical Officer Dr. Paul Burton appeared on CBS’ Face the Nation back in early May talking about expected efficacy of the shots. In the six months to 23 months group, effectiveness is around 50%. For the two years to five years cohort, that falls to around 37%.
Morris says he anticipates adding more Moderna shots to the two-dose regimen in place now.
“I would likely anticipate it would be a three-shot. In other words, do two and then a while later do another booster, much like we’re doing with adults,” he says.
Morris believes that if that adjustment does happen, it could make the vaccines more palatable to a greater percentage of parents who want to see more consistent results than what has been reported to date.
The Moderna data is more complete than what Pfizer presented. The tables below are pulled directly from Pfizer’s Emergency Use Authorization application submitted to the FDA’s Vaccines and Related Biological Products Advisory Committee. VRBPAC had to approve the application first, followed by the FDA as a whole and then the CDC.
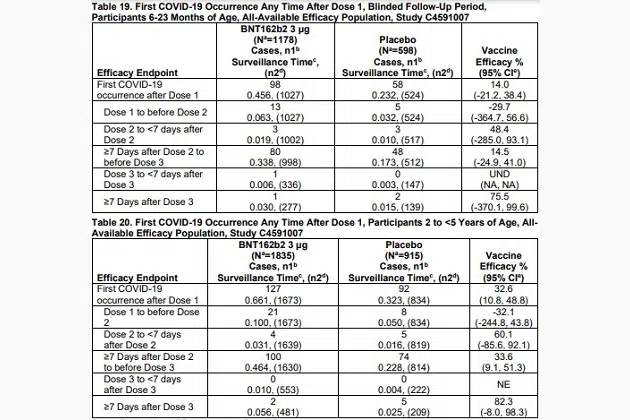
Pfizer says that efficacy should only be measured between its vaccine and a placebo that was offered during the double-blind trial as it pertains to COVID infections that happen over a week after the third dose is given. The company says that is when children meet the definition of full immunity.
For the 6-23 months age group, there are just three recorded infections in that time period. For the two years to five years group, the sample size is seven. It is difficult, nearly impossible, to determine whether the results observed during the trials would match what happens in the real world with samples so small. To try, Pfizer calculated confidence intervals for the efficacy rate of its vaccines. Both of those ranges include negative values, meaning that the shots could have no effectiveness.
Several members of the panels sifting through the data mentioned this point, but they unanimously approved both shots even with their concerns. Dr. Morris was asked whether he thought regulators were exercising appropriate oversight or if they were treating the pharmaceutical companies in a generous manner.
Morris says there are two aspects when answering the question that gives him confidence in the process. The first is existing data points from other vaccines. Modifications have to be made for younger children, but Morris points out we know how to do that and have already made that leap somewhat by providing shots for 5- to 11-year-olds. The second point is that the pharmaceutical companies have their data analyzed and then presented by independent experts who are required to disclose any potential conflicts of interest. The panels are hearing from companies as well as unbiased sources to get a complete picture.
“I believe the conversations are happening and the hard questions are being asked of the vaccine experts and the public health experts. And we are getting good answers. And I encourage them to do that even more as we think about what pandemic is next,” Morris says.
COVID-19 is the first pandemic since Swine Flu in 2009 that reached American shores. Morris says there will be another one at some point and the process needs to shift from merely reacting to COVID-19 to looking at where things broke down. Morris hopes authorities will identify our vulnerabilities and bolster public health for the next time the system is tested.
Morris recommends the shots for both age groups. He says it is important to highlight the fact that there are diseases whose effects are mitigated thanks to vaccines. It’s not just COVID-19. He urges parents to ensure they have their children up to date on measles and other immunizations and to be vigilant so that a preventable outbreak does not happen.
___
Copyright 2022 Leighton Enterprises, Inc. All rights reserved. This material may not be broadcast, published, redistributed, or rewritten, in any way without consent.